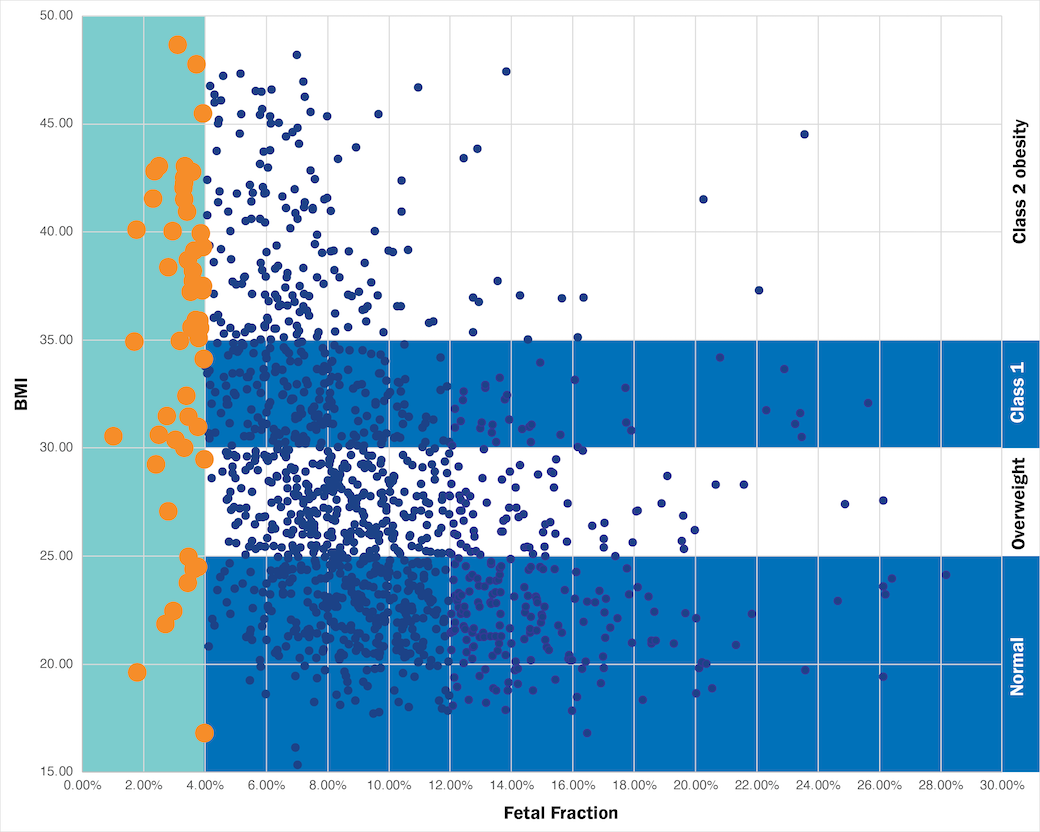
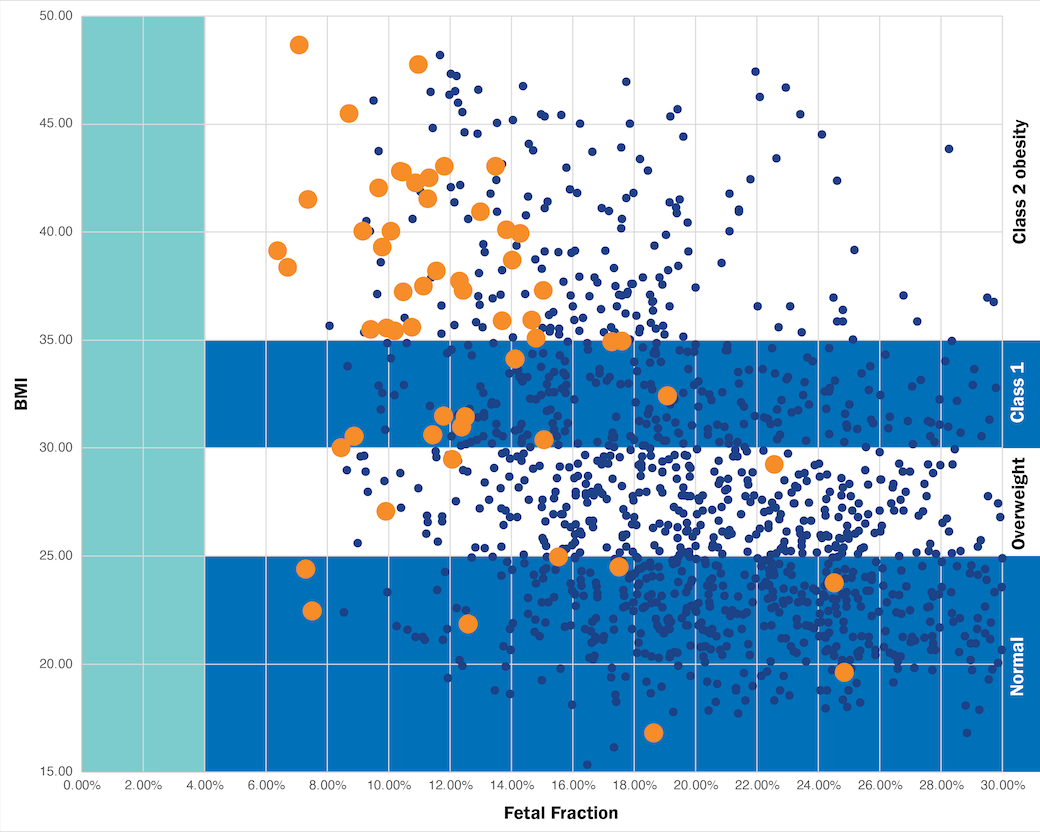
Only Prequel uses AMPLIFY™ Technology
AMPLIFY Technology makes Prequel the only prenatal cfDNA screen that delivers results that are 99.9% accurate for patients—on the first draw.5 By enriching fetal fraction, the Prequel Prenatal Screen improves sample performance for patients of all BMIs.6,7
How fetal fraction affects patient results
50%
24.3%
How AMPLIFY enhances results
3.9x
2.3x
Reduce inconclusive results with fetal fraction amplification
Move the slider from right to left to see how the Prequel Prenatal Screen with AMPLIFY enhances fetal fraction regardless of BMI. In particular, fetal fraction amplification helped patients who originally had less than 4% fetal fraction receive results with Prequel when they would have been at higher risk of inconclusive results with other screens.4,9
Screen for genetic risks sooner
The Prequel Prenatal Screen is the only cfDNA screen available at eight weeks’ gestation. In the rare event a baby is at risk of a genetic condition, the sooner your patients know, the more time they will have to pursue diagnostic testing and consult with specialists.4
Myriad compared Prequel to traditional cfDNA screens to see how soon fetal fraction amplification can provide results.
Prequel with AMPLIFY can achieve higher fetal fractions at eight weeks when compared to other screens at 10 weeks.4
Identify pregnancies at increased risk of complications
Provide more comprehensive care to your patients during pregnancy with Expanded Aneuploidy Analysis. It can help you assess if their pregnancies are at a higher risk for complications such as preterm birth, in addition to screening for genetic conditions that may affect their baby.10
1 in 200
Keep in mind… 1 in 200 pregnancies are at risk for a rare autosomal aneuploidy11
Screen for aneuploidy risks early
Through Prequel with Expanded Aneuploidy Analysis, you can identify aneuploidy risk sooner. This allows you to modify your patient’s monitoring plan, if needed.10, 11
Prequel screening options
Standard Panel Common Aneuploidies
- Trisomy 13 (Patau syndrome)
- Trisomy 18 (Edwards syndrome)
- Trisomy 21 (Down syndrome)
Available for singleton and twin pregnancy.
Sex Chromosome Analysis (Opt-in)
- Monosomy X (Turner syndrome)
- Klinefelter syndrome (XXY)
- Trisomy X (XXX)
- XYY syndrome
- Male (XY)
- Female (XX)
Available for singleton and twin pregnancy.
Microdeletions (Opt-in)
- 22q11.2 deletion (DiGeorge syndrome)
- 1p36 deletion syndrome
- 15q11.2 deletions (Angelman or Prader-Willi syndrome)
- 4p deletion (Wolf-Hirschhorn syndrome)
- 5p deletion (Cri-du-Chat syndrome)
Available for singleton pregnancy only.
Expanded Aneuploidy Analysis (Opt-in)
Expands aneuploidy analysis to include all 22 autosomes. Associated conditions include:
- Placental insufficiency (e.g. growth restriction, stillbirth)
- Uniparental disomy syndromes (e.g. Prader-Willi, Beckwith-Wiedemann)
- Fetal syndromes (e.g. trisomy 8, trisomy 22)
Available for singleton pregnancy only.
Trust in high positive predictive values
Prenatal diagnosis of 22q11.2 deletion syndrome (22q11.2 DS), also known as DiGeorge syndrome, can improve both pregnancy management and outcomes for newborns. The American College of Medical Genetics and Genomics (ACMG) recommended offering cfDNA screening for it, which had not been supported previously due to low positive predictive values. A recent study showed how the Prequel Prenatal Screen amplified fetal fraction, resulting in one of the industry’s highest reported positive predictive values for 22q11.2 DS.12 A positive result from the Prequel Prenatal Screen should be followed up with prenatal diagnosis.
Learn how Prequel provided up to 100% positive predictive values for 22q microdeletion detection
How enhanced screening technology benefits you
In addition to AMPLIFY Technology, the Prequel Prenatal Screen uses Whole genome sequencing (WGS). WGS has a higher sensitivity than Single nucleotide polymorphism (SNP), which is another form of cfDNA screening.7,13
Real-world performance (clinical experience)
| Metric | SNP | WGS |
| Overall sensitivity (common trisomies) | 95.5%13 | 98.6%7 |
| Affected pregnancies with inconclusive results | 7.5%13 | 0%7 |
| Failure rate | 2.55%*14 | 0.1%7 |
*Due to low fetal fraction only (excludes QC failures)
Who is the Prequel Prenatal Screen for?
See the types of patients who would be a good fit for the Prequel Prenatal Screen.

No significant risk
- Seeks baby’s predicted sex
- 1 in 200 chance of aneuploidy, despite absence of significant risk factors11

Sample failure risk
- Presents with high BMI
- At risk of low fetal fraction at eight weeks

High risk
A high-risk pregnancy due to:
- Age or prior pregnancies
- Family history


References:
- Dungan JS, et al. Noninvasive prenatal screening (NIPS) for fetal chromosome abnormalities in a general-risk population: An evidence-based clinical guideline of the American College of Medical Genetics and Genomics (ACMG). Genetics in Medicine. 2023;25(2):100336. doi:10.1016/j. gim.2022.11.004.
- Screening for fetal chromosomal abnormalities. Obstetrics & Gynecology. 2020;136(4). doi:10.1097/aog.0000000000004084.
- Committee Opinion No. 690 Summary: Carrier Screening in the Age of Genomic Medicine. 2017. Obstetrics & Gynecology. 129 (3): 595–96.
- Dugoff L. Fetal fraction amplification enables accurate prenatal cell-free DNA screening at 8 weeks gestation. Presented at: the Society for Maternal Fetal Medicine 2025 Pregnancy Meeting; January 30, 2025; Denver, CO.
- Welker NC, et al. High-throughput fetal fraction amplification increases analytical performance of noninvasive prenatal screening. Genetics in Medicine. 2021;23(3):443-450. doi:10.1038/s41436-020-01009-5.
- Yared E, et al. Obesity increases the risk of failure of noninvasive prenatal screening regardless of gestational age. Am J Obstet Gynecol. 2016;215(3):370.e1-370.e3706. doi:10.1016/j.ajog.2016.03.018.
- Hancock S, et al. Clinical experience across the fetal-fraction spectrum of a non-invasive prenatal screening approach with low test-failure rate. Ultrasound Obstet Gynecol. 2020;56(3):422-430. doi:10.1002/uog.21904.
- Deputy NP, et al. Prevalence and Trends in Prepregnancy Normal Weight - 48 States, New York City, and District of Columbia, 2011-2015. MMWR Morb Mortal Wkly Rep. 2018;66(51-52):1402-1407. Published 2018 Jan 5. doi:10.15585/mmwr.mm665152a3.
- Muzzey D, et al. Noninvasive prenatal screening for patients with high body mass index: Evaluating the impact of a customized whole genome sequencing workflow on sensitivity and residual risk. Prenat Diagn. 2020;40(3):333-341. doi:10.1002/pd.5603.
- Chawla D, et al. Screening Positive for Rare Autosomal Aneuploidies Increases Frequency of Adverse Pregnancy Outcomes and Alters Clinical Management. Prenat Diagn. 2025. doi/10.1002/pd.6776.
- Toutain J, et al. Confined placental mosaicism revisited: Impact on pregnancy characteristics and outcome. PLoS One. 2018;13(4):e0195905. doi:10.1371journal/pone.0195905.
- Hammer C, et al. High positive predictive value 22q11.2 microdeletion screening by prenatal cell-free DNA testing that incorporates fetal fraction amplification. Prenat Diagn. 2024; 44(8): 925-935. doi:10.1002/pd.6562.
- Dar P, et al. Cell-free DNA screening for trisomies 21, 18, and 13 in pregnancies at low and high risk for aneuploidy with genetic confirmation. Am J Obstet Gynecol. 2022;227(2):259.e1-259.e14. doi:10.1016/j.ajog.2022.01.019.
- Benn P, et al. Accuracy of fetal fraction measurements in a single-nucleotide polymorphism-based noninvasive prenatal test. Prenat Diagn. 2024;44(10):1218-1224. doi:10.1002/pd.6634
- Artieri CG, et al. Noninvasive prenatal screening at low fetal fraction: comparing whole-genome sequencing and single-nucleotide polymorphism methods. Prenat Diagn. 2017;37(5):482-490. doi:10.1002/pd.5036.
- See sneakpeek.com/publications for clinical studies
- Milot H, et al. Large scale follow-up research study: SneakPeek® Early Gender DNA Test 99.9% accurate for fetal sex by live-birth confirmation. Int J Pregn & Chi Birth. 2020;6(6):165‒168. DOI: 10.15406/ipcb.2020.06.00217.
What is fetal fraction?
Fetal fraction describes the amount of fetal DNA in a pregnant person’s blood sample. Fetal fraction plays an important role in providing conclusive cfDNA screening results to your patients. Low fetal fraction, which is more common in patients with high BMIs, can lead to inconclusive results.6,9